
Students may be interested in the even smaller particles of quantum physics-like quarks, bosons, neutrinos, and antineutrinos. To make a Bohr model of carbon, you must check the Periodic Table to see how many protons, neutrons, and electrons carbon has. Drawing a shell model diagram and an energy diagram for hydrogen, and then using the diagrams to calculate the energy required to excite an electron between different energy levels. They are intended to get players thinking about what they already know about elements. About Transcript Calculating electron energy for levels n1 to 3. General questions about the properties of elements assume standard temperature and pressure (helium is liquid below -268☌ and gold is a liquid above 1064 ☌). For this game, the most common isotopes of the chemical elements are used. This game presents a simple introduction to the Rutherford-Bohr model of the atom and the way we organize the elements. NS 5.8.2 - Develop an understanding of properties of matter Note: Using the Bohr model, determine the energy, in electron volts, of the photon produced when an electron in a hydrogen atom moves from the orbit with n 5 to the orbit with n 2. Any bond or intermolecular attraction that can be formed can be broken It is the amount of energy that an electron gains when subjected to a potential of 1 volt 1 eV 1.602 (×) 10 19 J.
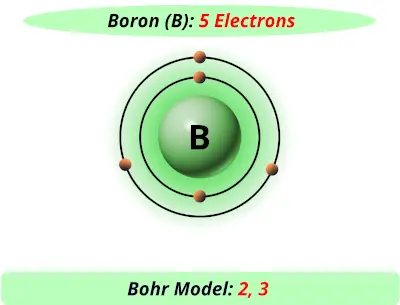
Chemical and physical properties of materials can be explained by the structure and arrangement of atoms, ions, and molecules and the forces between them.Matter is made from discrete fundamental units called atoms.Concepts:Ĭhemical elements, Periodic Table, chemical symbols, atom, atomic number, atomic weight, protons, electrons, neutrons, atomic structure. Interactive module that introduces atomic structure Learning objectives:Īfter this activity, the student will be able to describe the basic structure of matter, name the parts of an atom, have experience using the Periodic Table, explain elements, and have the background to understand isotopes. Teacher Information: Activity Description:


 0 kommentar(er)
0 kommentar(er)
